
At this condition, the water content by SRK EoS is 0.0012 mole fraction equivalent of 57 lb m/MMSCF or 914 kg/10 6 std.
#Hydrocarbon dew point calculator software free#
Due to the removal of free water and heavy hydrocarbons from mixture 2, the phase envelope and the water dew point curve have moved to the left, as expected.

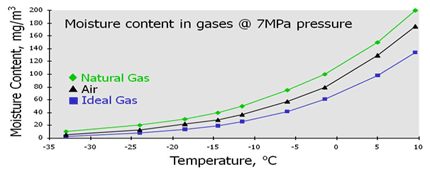
So, below about 1400 psia, the wet hydrocarbon dew point is predicted while for pressures above 1400 psia, the water dew point (the higher one) is predicted.įinally, mixture 2 has been passed through a separator at 100 ☏ and 1000 psia and the resulting vapor compositions from a three-phase flash calculation based on the SRK EoS is shown in the last column of Table 1 as mixture 4. Also note that at a specified pressure, the higher of the two dew points (hydrocarbon and water) have been calculated by the SRK EoS. It is interesting to note that the dry hydrocarbon dew points and the wet hydrocarbon dew points predicted by SRK coincide very closely with each other the difference is practically negligible. The compositions of natural gases studied in this work are shown in Table 1. This version of SRK is tailor fitted for water-hydrocarbon systems. In this work we will use Figure 6.1 of Volume 1 and the modified Soave-Redlich-Kwong (SRK EoS) reported in GPA RR-42 by Erbar et al. The details of these methods can be found in Chapter 6 of Volume 1 and Chapter 9 of Volume 3 of “Gas Conditioning and Processing”. There are several methods of calculating of water content. The water content of a gas depends on the system temperature, pressure and composition of the water containing gas.
In the past Tips of the Month, we discussed the phase behavior of water-free natural gas mixtures.


 0 kommentar(er)
0 kommentar(er)
